Blood and Blood Components
Meeting patients' needs for plasma-derivatives is an ongoing challenge. Canada is currently 100% self-sufficient in supply of whole-blood and fresh frozen plasma for transfusions (though donations are always required to keep it that way). However, altruistic donations of plasma for manufacturing of plasma-derivatives (that are used in treatment of patients with immunodeficiency and autoimmune diseases, bleeding disorders and other rare diseases) meet only 17% of the demand. The remainder of plasma products used in Canada are imported from commercial companies in the US and beyond who use paid donors. As such, Canada is one of the top users of processed plasma products from other countries.
The World Health Organization recommends the elimination of paid donations to ensure blood safety and availability; however, this recommendation is not always heeded. In 2013, Canadian Plasma Resourses’ (CPR), a private, for profit, organization planned to open pay-for-plasma clinics in Toronto and Hamilton. The company’s plans prompted public debate about the issue, and eventually, Ontario passed legislation banning compensation for blood and blood components. To date, Québec, Alberta, and British Columbia have similar legislation. Although the clinics in Ontario did not open, CPR has active clinics in Moncton and Saskatoon. On February 25th, 2018 CBC Radio One program The Sunday Edition hosted a debate about financial compensation (cas h payment) for plasma providers. On the debate, Dr. Sher (CEO, Canadian Blood Services) affirmed public concerns about the shortage of donations and explained that Canadian Blood Services’ plans to improve access, rather than incentivize participation through financial compensation. In his words, “the question is not should we or should we not rely on remunerated donors. We do today. Eighty-five per cent of the supply comes from the United States, where they pay their plasma donors. The issue in Canada is, how do we want to solve the domestic security of supply concern? …Do we want a public sector solution which guarantees security of supply for Canadians, or do we welcome private sector players into the Canadian market who will sell the plasma anywhere on the international market to the highest bidder?”
h payment) for plasma providers. On the debate, Dr. Sher (CEO, Canadian Blood Services) affirmed public concerns about the shortage of donations and explained that Canadian Blood Services’ plans to improve access, rather than incentivize participation through financial compensation. In his words, “the question is not should we or should we not rely on remunerated donors. We do today. Eighty-five per cent of the supply comes from the United States, where they pay their plasma donors. The issue in Canada is, how do we want to solve the domestic security of supply concern? …Do we want a public sector solution which guarantees security of supply for Canadians, or do we welcome private sector players into the Canadian market who will sell the plasma anywhere on the international market to the highest bidder?”
At the moment, laws on blood and plasma donations are regulated at two levels. Health Canada regulates the safety of donations, and the provincial governments regulate payment. However, that division could change. The Voluntary Blood Donations Act (An Act to amend the Blood Regulations) is currently passing through the senate. The Act proposes that in addition to further regulations laid out in the Blood Regulations: ‘An establishment, other than Canadian Blood Services, must not collect allogeneic blood for remuneration or benefit for the donor unless the blood collected is of a rare phenotype’. In effect, this act is an answer to Dr. Sher’s well-timed question. The act passed the Second Reading in the Senate on October 25, 2018, and is currently being reviewed by the Social Affairs Committee at the Senate. The regulatory text is open to the public for comment. Senator Pamela Wallin has spoken to the committee about the bill; if you are interested in commenting on the bill, submissions should be sent to:
- Senator Chantal Petitclerc: Chantal.Petitclerc@sen.parl.gc.ca
- Senator Pamela Wallin: pamela.wallin@sen.parl.gc.ca
For further information, view Senator Wallin on why she wants the Senate to support her bill.
Last updated Winter 2019
Timeline
|
1980s |
Tainted blood products, many of which came from paid at-risk populations in the United States, infected thousands of Canadians with HIV and Hepatitis C. |
The Cangene Corporation (1984-2014) facility opened a clinic in Winnipeg, paying “donors” with the extremely rare Rh-negative blood type. |
1984 |
|
|
1997 |
Krever Commission A national 'vein to vein' standard for blood was initiated by the Canadian Standards Association. |
Canadian Blood Services and Héma-Québec were created. A new licensing framework for blood establishments was introduced by Health Canada. |
1998 |
|
| 2006 | The Food and Drug Regulations were further amended to include provisions for human plasma collected by plasmapheresis. | |
Canadian Plasma Resources announced plans to open three clinics in Ontario – two in Toronto and one in Hamilton – at which it would pay “donors” for their plasma. |
Feb 2013 |
|
|
Feb 2014 |
The Cangene Corporation was acquired by US based ‘Emergent Biosolutions’and the plasma “donation” facility in Winnipeg closed. |
Following Bill 21, the Safeguarding Health Care Integrity Act, received Royal Assent. This bill, amongst other things, prohibits direct or indirect payment as well as receipt of payment for blood or blood components. |
Dec 2014 |
|
|
2016 |
Canadian Plasma Resource (private, for profit) opened a clinic in Saskatoon. |
Health Canada approved the Moncton CPR plasma collection centre. |
July 2017 |
|
| May 2018 | The Voluntary Blood Donations Act was introduced in the senate. |
In the 1980s, tainted blood products – many of which came from paid at-risk populations in the United States – infected thousands of Canadians with HIV and Hepatitis C. At the time, the federal government appointed a Royal Commission (known as the Krever Commission). In 1997, the Krever Commission recommended that Canada only accept payment for blood in rare circumstances. On this basis, Canadian Blood Services and Héma-Québec were created as arms-length agencies to administer the donation and provision of blood and blood components. Since then, blood donation within Canadian borders has been heavily screened. With the exception of the Cangene Corporation (1984-2014) facility in Winnipeg, which paid “donors” with the extremely rare Rh-negative blood type, blood donation in Canada remained altruistic.
Recent developments, however, have put Canada’s non-profit system under stress. In February 2013, Canadian Plasma Resources announced plans to open three clinics in Ontario – two in Toronto and one in Hamilton – at which it would pay “donors” for their plasma. One of these facilities was located next to a homeless shelter and another next to a drug-treatment facility. This raised concerns about the exploitation of at-risk individuals and about increased safety risks for recipients of paid-for blood.
In March 2013, the Ontario Minister of Health wrote to the federal Minister of Health [PDF - 89 KB] expressing concerns about the integrity of the altruistic blood donor system. A month later, Health Canada chaired a roundtable followed by a public consultation process (to which Matthew Herder, Françoise Baylis, and Jocelyn Downie contributed [PDF - 26 KB]). Notably, in announcing the consultation process, Health Canada overstated the scope of payment for blood in Canada by incorrectly asserting that “the practice of payment for donations of plasma for the creation of plasma-derived pharmaceuticals has occurred in Canada for 30 years.” In point of fact, at that time, payment for plasma donations only occurred for Rh-negative blood at the Cangene Corporation clinic in Winnipeg.
About a year later, in February 2014, Matthew Herder, Françoise Baylis, and Jocelyn Downie wrote to the Ontario Health Minister Deb Matthews [PDF - 2.9 MB], urging the government to amend section 10 of the Trillium Gift of Life Network Act in order to ban payment for blood and blood components. In due course, the Ontario government announced that it would ban payment for blood and plasma in the province. On December 11, 2014, Bill 21, the Safeguarding Health Care Integrity Act, received Royal Assent. This legislation empowers the Ontario government to control licensing for labs and specimen collection centers. It also distinguishes between plasma donations used in drug manufacturing and fresh blood and plasma used in transfusions. Schedule 1 includes provisions from Bill 178 (the Voluntary Blood Donations Act) that prohibit direct or indirect payment as well as receipt of payment for blood or blood components.
In February 2016, Canadian Plasma Resources a private, for-profit plasma collection company, opened a clinic in Saskatoon. A little more than a year later, it opened a second clinic in Moncton. Initially compensation was set at $25.00 per “donation.” Currently, compensation is $50.00 per “donation”. This money is credited to a Donor Value Card (a non-transferable VISA card) and qualified donors are limited to one “donation” per week. The company has reported that it hopes to open additional for-profit clinics in other provinces including Nova Scotia. In May 2018, the Voluntary Blood Donations Act, which prohibits remuneration of blood and blood component donors, was introduced to the senate. The bill has passed its second reading and is currently under review by the Social Affairs Committee in the senate. As the act gets closer to being enacted, it begins to jeopardize CPR’s operations in Canada.
The World Health Organization recommends the elimination of paid donations to ensure blood safety and availability. With limited research on the Canadian context, deciding how best to ensure a safe and sufficient supply that, at the same time, avoids the harms of exploitation means that policy choices must be made under conditions of uncertainty.
Following the 1997 Krever Commission recommendations, the altruistic blood donor system appears to have served Canadians well. That does not mean that all Canadians are happy with the system. Indeed, public responses to the CBC Radio One Programme The Sunday Edition hosted on February 25th, 2018 were largely critical of Canadian Blood Services. Concerns were expressed about problems of access and inconveniences now attached to donation. In an unscheduled session added the following week, host Michael Enright read a sample of the mail correspondence before asking Dr. Sher (CEO, Canaidan Blood Services) whether he was surprised that “people are so concerned about this?” Sher responded, saying: “This sends the opposite message to me. It indicates the very high level of support and commitment that Canadians have for their national blood service …this to me actually demonstrates how important Canadians view their blood system and I’m very, very proud that this many people would be interested in writing to you and saying we either have concerns, questions or ideas.” Canadians’ concerns about their blood system extend beyond whether payment should be permitted. However, some believe that these problems would be solved by introducing a paid, for-profit donation system.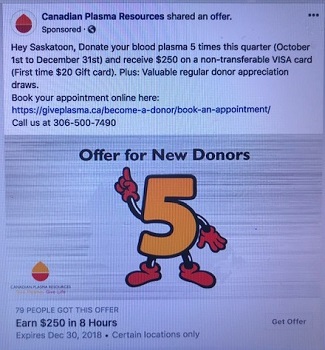
At the moment neither federal nor provincial legislation (outside of Québec, Ontario, Alberta and most recently British Columbia) actually ban compensation for blood or blood components (including plasma). Health Canada is reported by the CBC to have claimed that "its mandate is to regulate the safety and quality of the plasma that is collected for the purposes of transfusion or use in the manufacture of a human drug, which does not extend to corporate or operational decisions such as compensation to donors." Thus, for the time being, there is little to prohibit private for-profit companies in Canada from attempting to incentivize donation of blood or blood components through financial reimbursement schemes in those jurisdictions that lack relevant law and policy. This situation may not persist, as the Federal Government is currently debating the Voluntary Blood Donations Act, which would heavily restrict the payment of donors across Canada.
For more in depth coverage of many of these issues, consult the work of Anne Kingston in: "Should we pay for blood?" (Macleans Magazine, 15 Apr 2013), "What a blood plasma-for-profit clinic means for public health care" (Macleans Magazine, 14 Jan 2107), and "A bloody mess: the story behind paid plasma in Canada" (Macleans Magazine, 22 Nov 2017) as well as a response to the latter in the "Letter to the editor" by Graham Sher, Executive Director, Canadian Blood Services.
Opinion Pieces
- Wilson, A. (27 May 2018). Panel wrong about need to pay for plasma donations. The Star.
- Lanteigne, K. (6 Feb 2018). More funding needed now to increase blood plasma donations. TheStar.com.
- Raza, D. (1 Jan 2018). Safety of blood supply, not profits, should be Health Canada's top concern. TheStar.com.
- Desjardins, L. (22 Nov 2017). Close for-profit blood clinics, urge advocates. Radio Canada International.
- Smarsh, S. (Summer 2017). Blood brother: Blood is donated, pla$sma is purcha$ed. VQR: A National Journal of Literature & Discussion, 93(3).
- Mallick, H. (26 Apr 2016). Blood agency would turn us into stop ‘n’ shops: Mallick.Toronto Star.
- Anonymous. (19 Mar 2014). Ontario is right to ban paid donation of blood plasma: Editorial.Toronto Star.
- Rae, B. (7 Feb 2014). Why is it taking governments so long to say no to "pay for plasma"? www.bobrae.ca.
Legal Developments
- Young, I. (29 May 2018). Senator introducing bill to ban payments for blood donation. Global News.
- Bains, C. (26 Apr 2018). B.C. joins Alberta, Ontario, Quebec in banning payment for blood and plasma. CTV News.
- Kingston, A. (22 Nov 2017). A bloody mess: The story behind paid plasma in Canada. Macleans Magazine.
- Picard, A. (10 May 2016). It's beyond time for a clear policy on paying donors for plasma.The Globe and Mail.
- Crowe, K. (26 Apr 2016). Canadian Blood Services won't rule out paying plasma donors. CBC News.
- Babbage, M. (22 Jul 2014). Ontario bill proposes ban on paying for blood, blood plasma. The Canadian Press.
- Anonymous. (20 Jun 2013). 28 Organizations call for public policy review on for-profit plasma collection. Diablogue.
- Teotonio, I. (12 Apr 2013). Should federal and provincial regulators allow plasma-for-profit clinics to operate in Canada? The Star.
News About For Profit Clinics
- Greenberg, Zoe. (1 Feb 2019). What is the blood of a poor person worth. The New York Times.
- Bissett, K. (17 Jun 2016). Plans for paid-plasma donation clinic in New Brunswick raises ethical debate.The Brandon Sun.
- McMillan, E. (5 Apr 2016). Canadian plasma resources pays for donations, eyes Maritime expansion. CBC News.
- Brend, Y. (21 Mar 2016). Pay-for-plasma system looming in B.C. CBC News.
- Boon, J. (11 Mar 2016). For-profit plasma clinics exploit blood for profit say NDP. The Coast.
- Anonymous. (9 Mar 2016). Pay-for-plasma clinic may head to Nova Scotia. The Chronicle Herald.
- Graig, M. (18 Feb 2016). Controversial plasma collection clinic now open in Saskatoon. Global News.
- Grant, K. (24 Nov 2014). Company gives up on plan to open plasma clinics in Ontario. Globe & Mail.
- Blackwell, R. (12 Dec 2013). Cangene bought by US rival for $236 million, latest Canadian biotech taken private. The Globe and Mail.
Covering the National Debate on Payment
- Enright, M. (4 Mar 2018). Canadian blood services CEO responds to listener email. CBC Radio: The Sunday Edition.
- Enright, M. (25 Feb 2018). The debate over paying Canadians for plasma. CBC Radio: The Sunday Edition.
- Kingston, A. (22 Nov 2017). A bloody mess: The story behind paid plasma in Canada. Macleans Magazine.
- Dala, M., Doig, C., & Laupacis, A. (17 Apr 1014). Paying for plasma – Canada’s double standard? Healthy Debate.
- Kingston, A. (15 Apr 2013). Should we pay for blood? Maclean's.
Select Impact Ethics Blogs
- Getz, L.J. (26 Oct 2018). Thicker than water: Slow movement on blood policy. IMpact Ethics.
- Getz, L.J. (24 Aug 2017). A reflection on blood donation policy in Canada. Impact Ethics.
- Nielsen, L.W. (22 Apr 2016). Got plasma? Impact Ethics.
- Herder, M. (20 Apr 2016). Talking plasma and markets with my mothers. Impact Ethics.
- Behrmann, J., & Ravitsky, V. (30 Oct 2014). After plasma: Are blood stem cells next? Impact Ethics.
- Lanteigne, K. (21 Mar 2014). Blood money. Impact Ethics.
- Haw, J. (17 Oct 2013). Canada's national public cord blood bank opens its doors.Impact Ethics.
- Herder, M., & Baylis, F. (6 Aug 2013). Policy laundering and payment-for-plasma.Impact Ethics.
- Dutt, M. (31 Jul 2013). For-profit plasma clinics are risky business. Impact Ethics.
Select Journal Articles
- WHO. (1975 onwards). Resolutions relating to blood safety adopted by WHO governing bodies. World Health Organization.
- WHO. (Jun. 2015). Blood and blood safety availability. Fact sheet N°279. World Health Organization.
- Health Canada. (27 Jun. 2013). Backgrounder paper – plasma donations in Canada. Drugs and Health Products.
- Canadian Blood Services. (18 Feb 2016). Statement from Canadian Blood Services: Payment for Plasma Donation.
- Glauser, W. (28 Jul 2015). Payment for plasma raises ethical issues. Canadian Medical Association Journal, doi: 10.1503/cmaj.109-4855.
- Health Canada. (May 2014). Plasma Donation in Canada—Fact Sheet.
- WHO & IFRCRCS. (2010). Towards 100% voluntary blood donation: A global framework for action [PDF 7.79 MB]. World Health Organization & International Federation of Red Cross and Red Crescent Socieities.
Select Policy Contributions
Baylis, F., Downie, J., & Herder, M. (2013). Round table discussion on payment of plasma donors in Canada - Summary report [PDF - 29.5KB]. Submitted to: Office of Policy and International Collaboration (BGDT).
Overview
Meeting patients' needs for plasma-derivatives is an ongoing challenge. Canada is currently 100% self-sufficient in supply of whole-blood and fresh frozen plasma for transfusions (though donations are always required to keep it that way). However, altruistic donations of plasma for manufacturing of plasma-derivatives (that are used in treatment of patients with immunodeficiency and autoimmune diseases, bleeding disorders and other rare diseases) meet only 17% of the demand. The remainder of plasma products used in Canada are imported from commercial companies in the US and beyond who use paid donors. As such, Canada is one of the top users of processed plasma products from other countries.
The World Health Organization recommends the elimination of paid donations to ensure blood safety and availability; however, this recommendation is not always heeded. In 2013, Canadian Plasma Resourses’ (CPR), a private, for profit, organization planned to open pay-for-plasma clinics in Toronto and Hamilton. The company’s plans prompted public debate about the issue, and eventually, Ontario passed legislation banning compensation for blood and blood components. To date, Québec, Alberta, and British Columbia have similar legislation. Although the clinics in Ontario did not open, CPR has active clinics in Moncton and Saskatoon. On February 25th, 2018 CBC Radio One program The Sunday Edition hosted a debate about financial compensation (cas h payment) for plasma providers. On the debate, Dr. Sher (CEO, Canadian Blood Services) affirmed public concerns about the shortage of donations and explained that Canadian Blood Services’ plans to improve access, rather than incentivize participation through financial compensation. In his words, “the question is not should we or should we not rely on remunerated donors. We do today. Eighty-five per cent of the supply comes from the United States, where they pay their plasma donors. The issue in Canada is, how do we want to solve the domestic security of supply concern? …Do we want a public sector solution which guarantees security of supply for Canadians, or do we welcome private sector players into the Canadian market who will sell the plasma anywhere on the international market to the highest bidder?”
h payment) for plasma providers. On the debate, Dr. Sher (CEO, Canadian Blood Services) affirmed public concerns about the shortage of donations and explained that Canadian Blood Services’ plans to improve access, rather than incentivize participation through financial compensation. In his words, “the question is not should we or should we not rely on remunerated donors. We do today. Eighty-five per cent of the supply comes from the United States, where they pay their plasma donors. The issue in Canada is, how do we want to solve the domestic security of supply concern? …Do we want a public sector solution which guarantees security of supply for Canadians, or do we welcome private sector players into the Canadian market who will sell the plasma anywhere on the international market to the highest bidder?”
At the moment, laws on blood and plasma donations are regulated at two levels. Health Canada regulates the safety of donations, and the provincial governments regulate payment. However, that division could change. The Voluntary Blood Donations Act (An Act to amend the Blood Regulations) is currently passing through the senate. The Act proposes that in addition to further regulations laid out in the Blood Regulations: ‘An establishment, other than Canadian Blood Services, must not collect allogeneic blood for remuneration or benefit for the donor unless the blood collected is of a rare phenotype’. In effect, this act is an answer to Dr. Sher’s well-timed question. The act passed the Second Reading in the Senate on October 25, 2018, and is currently being reviewed by the Social Affairs Committee at the Senate. The regulatory text is open to the public for comment. Senator Pamela Wallin has spoken to the committee about the bill; if you are interested in commenting on the bill, submissions should be sent to:
- Senator Chantal Petitclerc: Chantal.Petitclerc@sen.parl.gc.ca
- Senator Pamela Wallin: pamela.wallin@sen.parl.gc.ca
For further information, view Senator Wallin on why she wants the Senate to support her bill.
Last updated Winter 2019
History
Timeline
|
1980s |
Tainted blood products, many of which came from paid at-risk populations in the United States, infected thousands of Canadians with HIV and Hepatitis C. |
The Cangene Corporation (1984-2014) facility opened a clinic in Winnipeg, paying “donors” with the extremely rare Rh-negative blood type. |
1984 |
|
|
1997 |
Krever Commission A national 'vein to vein' standard for blood was initiated by the Canadian Standards Association. |
Canadian Blood Services and Héma-Québec were created. A new licensing framework for blood establishments was introduced by Health Canada. |
1998 |
|
| 2006 | The Food and Drug Regulations were further amended to include provisions for human plasma collected by plasmapheresis. | |
Canadian Plasma Resources announced plans to open three clinics in Ontario – two in Toronto and one in Hamilton – at which it would pay “donors” for their plasma. |
Feb 2013 |
|
|
Feb 2014 |
The Cangene Corporation was acquired by US based ‘Emergent Biosolutions’and the plasma “donation” facility in Winnipeg closed. |
Following Bill 21, the Safeguarding Health Care Integrity Act, received Royal Assent. This bill, amongst other things, prohibits direct or indirect payment as well as receipt of payment for blood or blood components. |
Dec 2014 |
|
|
2016 |
Canadian Plasma Resource (private, for profit) opened a clinic in Saskatoon. |
Health Canada approved the Moncton CPR plasma collection centre. |
July 2017 |
|
| May 2018 | The Voluntary Blood Donations Act was introduced in the senate. |
In the 1980s, tainted blood products – many of which came from paid at-risk populations in the United States – infected thousands of Canadians with HIV and Hepatitis C. At the time, the federal government appointed a Royal Commission (known as the Krever Commission). In 1997, the Krever Commission recommended that Canada only accept payment for blood in rare circumstances. On this basis, Canadian Blood Services and Héma-Québec were created as arms-length agencies to administer the donation and provision of blood and blood components. Since then, blood donation within Canadian borders has been heavily screened. With the exception of the Cangene Corporation (1984-2014) facility in Winnipeg, which paid “donors” with the extremely rare Rh-negative blood type, blood donation in Canada remained altruistic.
Recent developments, however, have put Canada’s non-profit system under stress. In February 2013, Canadian Plasma Resources announced plans to open three clinics in Ontario – two in Toronto and one in Hamilton – at which it would pay “donors” for their plasma. One of these facilities was located next to a homeless shelter and another next to a drug-treatment facility. This raised concerns about the exploitation of at-risk individuals and about increased safety risks for recipients of paid-for blood.
In March 2013, the Ontario Minister of Health wrote to the federal Minister of Health [PDF - 89 KB] expressing concerns about the integrity of the altruistic blood donor system. A month later, Health Canada chaired a roundtable followed by a public consultation process (to which Matthew Herder, Françoise Baylis, and Jocelyn Downie contributed [PDF - 26 KB]). Notably, in announcing the consultation process, Health Canada overstated the scope of payment for blood in Canada by incorrectly asserting that “the practice of payment for donations of plasma for the creation of plasma-derived pharmaceuticals has occurred in Canada for 30 years.” In point of fact, at that time, payment for plasma donations only occurred for Rh-negative blood at the Cangene Corporation clinic in Winnipeg.
About a year later, in February 2014, Matthew Herder, Françoise Baylis, and Jocelyn Downie wrote to the Ontario Health Minister Deb Matthews [PDF - 2.9 MB], urging the government to amend section 10 of the Trillium Gift of Life Network Act in order to ban payment for blood and blood components. In due course, the Ontario government announced that it would ban payment for blood and plasma in the province. On December 11, 2014, Bill 21, the Safeguarding Health Care Integrity Act, received Royal Assent. This legislation empowers the Ontario government to control licensing for labs and specimen collection centers. It also distinguishes between plasma donations used in drug manufacturing and fresh blood and plasma used in transfusions. Schedule 1 includes provisions from Bill 178 (the Voluntary Blood Donations Act) that prohibit direct or indirect payment as well as receipt of payment for blood or blood components.
In February 2016, Canadian Plasma Resources a private, for-profit plasma collection company, opened a clinic in Saskatoon. A little more than a year later, it opened a second clinic in Moncton. Initially compensation was set at $25.00 per “donation.” Currently, compensation is $50.00 per “donation”. This money is credited to a Donor Value Card (a non-transferable VISA card) and qualified donors are limited to one “donation” per week. The company has reported that it hopes to open additional for-profit clinics in other provinces including Nova Scotia. In May 2018, the Voluntary Blood Donations Act, which prohibits remuneration of blood and blood component donors, was introduced to the senate. The bill has passed its second reading and is currently under review by the Social Affairs Committee in the senate. As the act gets closer to being enacted, it begins to jeopardize CPR’s operations in Canada.
Payment Debate
The World Health Organization recommends the elimination of paid donations to ensure blood safety and availability. With limited research on the Canadian context, deciding how best to ensure a safe and sufficient supply that, at the same time, avoids the harms of exploitation means that policy choices must be made under conditions of uncertainty.
Following the 1997 Krever Commission recommendations, the altruistic blood donor system appears to have served Canadians well. That does not mean that all Canadians are happy with the system. Indeed, public responses to the CBC Radio One Programme The Sunday Edition hosted on February 25th, 2018 were largely critical of Canadian Blood Services. Concerns were expressed about problems of access and inconveniences now attached to donation. In an unscheduled session added the following week, host Michael Enright read a sample of the mail correspondence before asking Dr. Sher (CEO, Canaidan Blood Services) whether he was surprised that “people are so concerned about this?” Sher responded, saying: “This sends the opposite message to me. It indicates the very high level of support and commitment that Canadians have for their national blood service …this to me actually demonstrates how important Canadians view their blood system and I’m very, very proud that this many people would be interested in writing to you and saying we either have concerns, questions or ideas.” Canadians’ concerns about their blood system extend beyond whether payment should be permitted. However, some believe that these problems would be solved by introducing a paid, for-profit donation system.
At the moment neither federal nor provincial legislation (outside of Québec, Ontario, Alberta and most recently British Columbia) actually ban compensation for blood or blood components (including plasma). Health Canada is reported by the CBC to have claimed that "its mandate is to regulate the safety and quality of the plasma that is collected for the purposes of transfusion or use in the manufacture of a human drug, which does not extend to corporate or operational decisions such as compensation to donors." Thus, for the time being, there is little to prohibit private for-profit companies in Canada from attempting to incentivize donation of blood or blood components through financial reimbursement schemes in those jurisdictions that lack relevant law and policy. This situation may not persist, as the Federal Government is currently debating the Voluntary Blood Donations Act, which would heavily restrict the payment of donors across Canada.
For more in depth coverage of many of these issues, consult the work of Anne Kingston in: "Should we pay for blood?" (Macleans Magazine, 15 Apr 2013), "What a blood plasma-for-profit clinic means for public health care" (Macleans Magazine, 14 Jan 2107), and "A bloody mess: the story behind paid plasma in Canada" (Macleans Magazine, 22 Nov 2017) as well as a response to the latter in the "Letter to the editor" by Graham Sher, Executive Director, Canadian Blood Services.
Media Articles
Opinion Pieces
- Wilson, A. (27 May 2018). Panel wrong about need to pay for plasma donations. The Star.
- Lanteigne, K. (6 Feb 2018). More funding needed now to increase blood plasma donations. TheStar.com.
- Raza, D. (1 Jan 2018). Safety of blood supply, not profits, should be Health Canada's top concern. TheStar.com.
- Desjardins, L. (22 Nov 2017). Close for-profit blood clinics, urge advocates. Radio Canada International.
- Smarsh, S. (Summer 2017). Blood brother: Blood is donated, pla$sma is purcha$ed. VQR: A National Journal of Literature & Discussion, 93(3).
- Mallick, H. (26 Apr 2016). Blood agency would turn us into stop ‘n’ shops: Mallick.Toronto Star.
- Anonymous. (19 Mar 2014). Ontario is right to ban paid donation of blood plasma: Editorial.Toronto Star.
- Rae, B. (7 Feb 2014). Why is it taking governments so long to say no to "pay for plasma"? www.bobrae.ca.
Legal Developments
- Young, I. (29 May 2018). Senator introducing bill to ban payments for blood donation. Global News.
- Bains, C. (26 Apr 2018). B.C. joins Alberta, Ontario, Quebec in banning payment for blood and plasma. CTV News.
- Kingston, A. (22 Nov 2017). A bloody mess: The story behind paid plasma in Canada. Macleans Magazine.
- Picard, A. (10 May 2016). It's beyond time for a clear policy on paying donors for plasma.The Globe and Mail.
- Crowe, K. (26 Apr 2016). Canadian Blood Services won't rule out paying plasma donors. CBC News.
- Babbage, M. (22 Jul 2014). Ontario bill proposes ban on paying for blood, blood plasma. The Canadian Press.
- Anonymous. (20 Jun 2013). 28 Organizations call for public policy review on for-profit plasma collection. Diablogue.
- Teotonio, I. (12 Apr 2013). Should federal and provincial regulators allow plasma-for-profit clinics to operate in Canada? The Star.
News About For Profit Clinics
- Greenberg, Zoe. (1 Feb 2019). What is the blood of a poor person worth. The New York Times.
- Bissett, K. (17 Jun 2016). Plans for paid-plasma donation clinic in New Brunswick raises ethical debate.The Brandon Sun.
- McMillan, E. (5 Apr 2016). Canadian plasma resources pays for donations, eyes Maritime expansion. CBC News.
- Brend, Y. (21 Mar 2016). Pay-for-plasma system looming in B.C. CBC News.
- Boon, J. (11 Mar 2016). For-profit plasma clinics exploit blood for profit say NDP. The Coast.
- Anonymous. (9 Mar 2016). Pay-for-plasma clinic may head to Nova Scotia. The Chronicle Herald.
- Graig, M. (18 Feb 2016). Controversial plasma collection clinic now open in Saskatoon. Global News.
- Grant, K. (24 Nov 2014). Company gives up on plan to open plasma clinics in Ontario. Globe & Mail.
- Blackwell, R. (12 Dec 2013). Cangene bought by US rival for $236 million, latest Canadian biotech taken private. The Globe and Mail.
Covering the National Debate on Payment
- Enright, M. (4 Mar 2018). Canadian blood services CEO responds to listener email. CBC Radio: The Sunday Edition.
- Enright, M. (25 Feb 2018). The debate over paying Canadians for plasma. CBC Radio: The Sunday Edition.
- Kingston, A. (22 Nov 2017). A bloody mess: The story behind paid plasma in Canada. Macleans Magazine.
- Dala, M., Doig, C., & Laupacis, A. (17 Apr 1014). Paying for plasma – Canada’s double standard? Healthy Debate.
- Kingston, A. (15 Apr 2013). Should we pay for blood? Maclean's.
Further Reading
Select Impact Ethics Blogs
- Getz, L.J. (26 Oct 2018). Thicker than water: Slow movement on blood policy. IMpact Ethics.
- Getz, L.J. (24 Aug 2017). A reflection on blood donation policy in Canada. Impact Ethics.
- Nielsen, L.W. (22 Apr 2016). Got plasma? Impact Ethics.
- Herder, M. (20 Apr 2016). Talking plasma and markets with my mothers. Impact Ethics.
- Behrmann, J., & Ravitsky, V. (30 Oct 2014). After plasma: Are blood stem cells next? Impact Ethics.
- Lanteigne, K. (21 Mar 2014). Blood money. Impact Ethics.
- Haw, J. (17 Oct 2013). Canada's national public cord blood bank opens its doors.Impact Ethics.
- Herder, M., & Baylis, F. (6 Aug 2013). Policy laundering and payment-for-plasma.Impact Ethics.
- Dutt, M. (31 Jul 2013). For-profit plasma clinics are risky business. Impact Ethics.
Select Journal Articles
- WHO. (1975 onwards). Resolutions relating to blood safety adopted by WHO governing bodies. World Health Organization.
- WHO. (Jun. 2015). Blood and blood safety availability. Fact sheet N°279. World Health Organization.
- Health Canada. (27 Jun. 2013). Backgrounder paper – plasma donations in Canada. Drugs and Health Products.
- Canadian Blood Services. (18 Feb 2016). Statement from Canadian Blood Services: Payment for Plasma Donation.
- Glauser, W. (28 Jul 2015). Payment for plasma raises ethical issues. Canadian Medical Association Journal, doi: 10.1503/cmaj.109-4855.
- Health Canada. (May 2014). Plasma Donation in Canada—Fact Sheet.
- WHO & IFRCRCS. (2010). Towards 100% voluntary blood donation: A global framework for action [PDF 7.79 MB]. World Health Organization & International Federation of Red Cross and Red Crescent Socieities.
Select Policy Contributions
Baylis, F., Downie, J., & Herder, M. (2013). Round table discussion on payment of plasma donors in Canada - Summary report [PDF - 29.5KB]. Submitted to: Office of Policy and International Collaboration (BGDT).

