Positive electrode materials
High capacity materials
High capacity materials are considered the next generation of positive electrode materials for Li-ion batteries, and are starting to emerge in both commercial and industrial applications. Their synthetic, structural, electrochemical, and safety properties are studied extensively using a variety of characterization techniques, including XRD, SEM, and galvanostatic cycling.

Synthesis
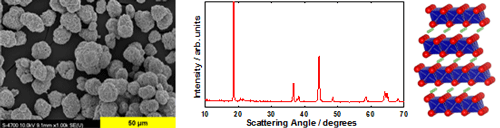
Structure and Morphology
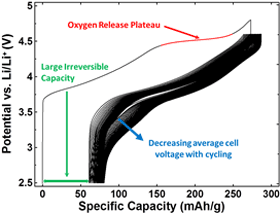
Electrochemistry
The lithium-rich layered metal oxides are currently the highest-capacity positive electrode materials under study1, exhibiting large irreversible capacity losses during their first cycle, voltage decay with extended cycling, and high reactivity with electrolyte when fully charged. Materials under study included single and multiphase compositions within the Li-Mn-Ni-O and Li-Mn-Ni-Co-O systems. Doped variants of these materials containing Al, Mg and other metals are also studied.
Core-shell materials
Lithium-rich layered Ni-Mn-Co oxide materials have been intensely studied in the last decade. Mn-rich materials have serious voltage fade issues and the Ni-rich materials have poor thermal stability and readily oxidize the organic carbonate electrolyte. Core-shell (CS) strategies that use Ni-rich material as the core and Mn-rich materials as the shell can balance the pros and cons of these materials in a hybrid system. Figure 1. shows cross-sectional SEM images and EDS maps of the CS precursors and lithiated samples. Figures 1b and 1c show a clear manganese and cobalt-rich shell and a nickel-rich core in the precursor. However, after sintering at 900oC, the cobalt rich shell disappeared (Figures 1B, 1C) and an approximately uniform distribution of cobalt across the particles was observed. This indicates there is inter-diffusion of the transition metals between the core phase and shell phase during sintering. A detailed study of the impact of inter-diffusion phenomena can guide the design of core-shell materials.
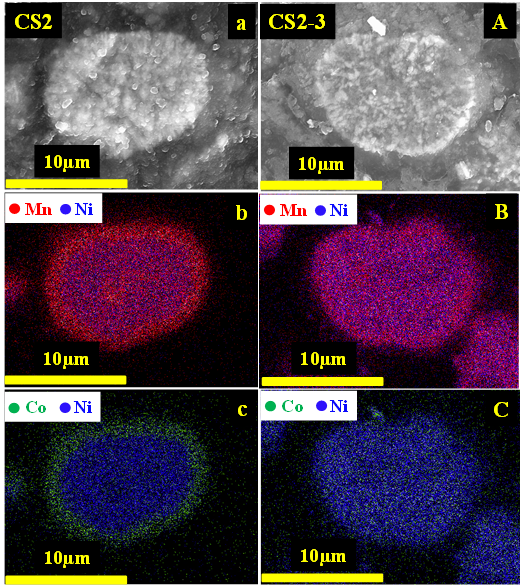
Figure 1 - SEM image of the cross-section of a core-shell precursor particle (a) and EDS mapping results (b, c). SEM image of the cross-section of a lithiated core-shell particle (A) and EDS mapping results (B, C).
References:
1. Li, J.; Camardese, J.; Shunmugasundaram, R.; Glazier, S.; Lu, Z.; Dahn, J. R. Synthesis and Characterization of the Lithium-Rich Core–Shell Cathodes with Low Irreversible Capacity and Mitigated Voltage Fade. Chem. Mater. 2015, 27, 3366–3377.
2. Camardese, J.; Li, J.; Abarbanel, D. W.; Wright, A. T. B.; Dahn, J. R. The Effect of Lithium Content and Core to Shell Ratio on Structure and Electrochemical Performance of Core-Shell Li(1+x)[Ni0.6Mn0.4](1-x)O2·Li(1+y)[Ni0.2Mn0.8](1-y)O2 Positive Electrode Materials. J. Electrochem. Soc. 2014, 162, A269–A277.
3. Li, J.; Doig, R.; Camardese, J.; Plucknett, K.; Dahn, J. R.; Measurements of Interdiffusion Coefficients of Transition Metals in Layered Li-Ni-Mn-Co Oxide Core-Shell Materials during Sintering. 2015, 17, 7765-7773.
